Launching a supplement brand takes more than eye-catching packaging — your label must meet strict FDA supplement labeling requirements. From layout and language to the Supplement Facts panel, every detail matters. Getting it wrong could mean delays, penalties, or lost trust with your customers.
In this guide, we’ll break down the FDA’s dietary supplement labeling regulations, formatting essentials, and practical tips to design a compliant, professional label that strengthens your brand in the market.
What Makes a Product a Dietary Supplement?
Before we delve into FDA supplement labeling regulations, it’s essential to understand what the FDA considers a dietary supplement.
According to the regulatory body, a dietary supplement is a product that contains one or more of the following ingredients: a vitamin, a mineral, an herb or other botanical, an amino acid, a dietary substance for use by man to supplement the diet by increasing the total dietary intake, or a concentrate, metabolite, constituent, extract, or a combination of any ingredient mentioned above.
These details must appear either on the front label panel or the information panel.
FDA Supplement Labeling Regulations
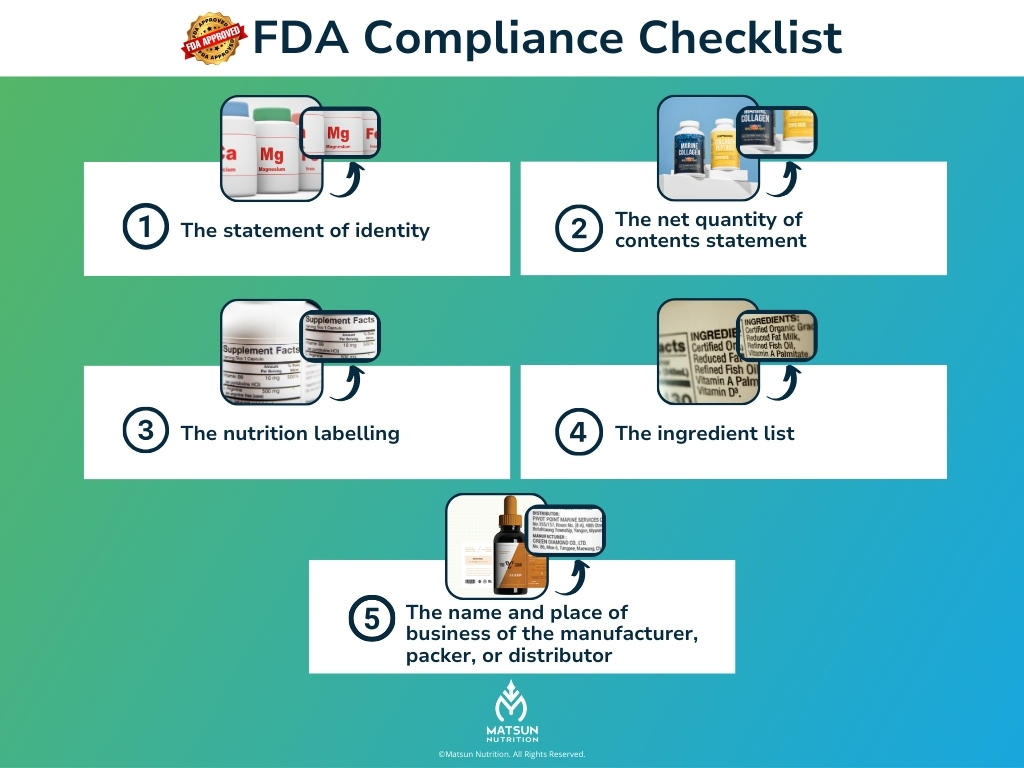
The FDA requires the following information on all dietary supplement labels:
- The statement of identity (name of the dietary supplement)
- The net quantity of contents statement (amount of the dietary supplement)
- The nutrition labeling
- The ingredient list
- The name and place of business of the manufacturer, packer, or distributor
These details must appear either on the front label panel or information panel.
Front Label Panel
The front label or principal display panel is the side of the label consumers are most likely to notice first as they browse the store shelves. The principal display panel must contain the following details:
- The statement of identity: This is simply your brand’s official name. If it doesn’t have a common name, you must clearly describe your supplement’s purpose.’
- The net quantity of contents statement: This informs consumers about the amount of the supplement in the container. You must indicate it in a standard unit of measure, such as grams, milligrams, ounces, or milliliters.
Informational Panel
The informational panel is the part of the label that’s typically located to the right of the front label panel. It includes more technical product details, such as:
- The nutrition labeling: This is the heart of your label. Commonly known as the supplements facts section, this part includes the supplement serving size, the amount of each ingredient per serving, and the Daily Value (DV) for certain vitamins and minerals.
- The ingredient list: This section provides a detailed breakdown of all the ingredients in your supplement, listed in descending order by weight.
- The name and place of business of the manufacturer, packer, or distributor: This section identifies the company responsible for bringing the supplement to market. It should include the full legal name and address of the manufacturer, packer, or distributor.
FDA Font and Readability Requirements

A well-designed supplement label goes beyond including comprehensive product details. It should also provide information in a way that’s easy for consumers to understand. As a result, the FDA also has supplement labeling guidelines focusing on label legibility. Here’s what you need to keep in mind:
- Font size: You must use a minimum font size of 1/16th of an inch for all information on the label except the front label display — using the lowercase ”o” as a baseline. Additionally, the height of letters should not be more than three times their width to maintain readability.
- Color contrast: Your label design shouldn’t overshadow the essential information. Your font color must contrast well with the background color.
While the FDA requires companies to adhere to these text formatting requirements, sometimes sticking to them is impractical. As a result, the FDA allows supplement manufacturers to petition for exemptions.
However, filing for an exemption requires justification and approval, so it’s best to prioritize clear wording whenever possible.
Designing an Effective Supplement Label

Complying with FDA regulations is just one piece of the puzzle. If you want your supplement brand to sell in high volumes, your label product must attract consumer’s attention.
According to a report by the Ehrenberg-Bass Institute of Marketing, consumers spend about 19 seconds making purchasing decisions when shopping online. Meanwhile, when shopping at brick-and-mortar stores, this figure drops to 13 seconds.
So, how can you attract consumer attention in just a matter of seconds? Here are tips for designing dietary supplement labels that will capture the attention of consumers:
Use a Clear and Concise Layout
Consumers scan countless labels in a short period, whether they’re shopping online or at physical stores. Make your product stand out with a clear and organized layout.
Group related information together, use bullet points for lists, and separate sections with subtle lines or different colors. This visual hierarchy will enable consumers to scan your product easily and retrieve the information they need quickly.
Use Appropriate Colors
Color plays a significant role in capturing consumer’s attention and influencing their purchasing decisions. Using suitable colors can be an excellent way to evoke certain emotions and reinforce your brand identity.
For instance, green often represents health and vitality, making it ideal for various herbal supplements and organic products. On the other hand, gold often represents quality and luxury products, making it suitable for high-end supplements.
Research different color associations and choose a palette that aligns with your product’s benefits.
Use High-Quality Visuals
Your label speaks volumes about your product. Use high-quality materials and printing to show off your brand’s quality. Cutting corners on your label could save costs upfront but at the expense of your product’s perceived value, potentially denting your sales.
Use High-Quality Materials
Your label speaks volumes about your product. Use high-quality materials and printing to show off your brand’s quality. Cutting corners on your label could save costs upfront, but at the expense of your product’s perceived value, potentially denting your sales.
Maintain Brand Consistency
Your supplement packaging and label is an extension of your brand. Ensure you use the same brand colors, fonts, and logo to create a cohesive visual identity. If you provide multiple products, this can make it easier for consumers to identify your products, building brand recognition and fostering consumer trust.
Use Eco-Friendly Labels
Many consumers care about the environmental impact of their purchases. According to one study, 78% of American consumers stated that sustainability matters to them. Furthermore, over 60% of consumers said that they would pay more for a product with sustainable packaging.
Using tree-free labels made from cotton, bamboo, or recycled fibers can show consumers you’re committed to sustainable practices.
Hire Graphic Designers
If implementing all these tips feels overwhelming, don’t worry. Hiring a graphic designer can enable you to create an attractive supplement label design that not only adheres to FDA regulations but also draws the attention of consumers.
Get an FDA-Approved Supplement Label
Creating an FDA-compliant supplement label can protect your business from costly legal consequences and penalties. However, regulatory compliance isn’t the only factor you should prioritize in your label design. Besides compliance, it’s essential to follow design best practices to ensure consumers take notice of your brand and choose it over others.
At Matsun Nutrition, our team is knowledgeable on the FDA’s regulations regarding supplement labels and we offer a variety of customized label designs. Contact us today to learn more about how we can help you craft eye-catching labels that can set your brand apart from competitors.
Build a Trusted Supplement Brand with FDA-Compliant Labels | Get Expert Guidance Today
Frequently Asked Questions (FAQs)
The FDA can take various actions against non-compliant labels, ranging from warning letters to product seizures and recalls. In extreme cases, you could also face fines. This can be damaging to your business and brand reputation. Consider working with a professional supplement label designer conversant with FDA labeling requirements to avoid such issues.
FDA regulations strictly prohibit the use of health claims on supplement labels. You cannot claim your product cures, treats, or prevents any disease. However, you can make structure or function claims based on established science, provided you have the evidence to support them.
It's best to consult the FDA guidelines for approved health claims or seek legal advice before using any health claims on your label.
While a lawyer can provide specific legal advice, it isn't always necessary. The FDA offers extensive resources and guidance documents to help you understand the labeling requirements.
However, if you have complex questions or concerns, consulting a lawyer or a supplement label manufacturer conversant with FDA regulations is a good idea.
Yes, different countries have different labeling requirements. If you intend to sell your supplements across various countries, you'll need to research the specific requirements for each target market. Consulting with a regulatory affairs specialist can ensure your labels comply with international regulations.
The FDA periodically updates its labeling guidelines. It's essential to stay abreast of any updates to ensure your brand remains compliant. You can subscribe to FDA updates on their website or partner with a supplement label company that monitors regulatory changes.



