With consumers seeking alternative ways to improve their health and well-being, the dietary supplement industry has witnessed significant growth over the years. As a result, many entrepreneurs are venturing into the business of creating their own supplement brands.
However, making your own supplement company requires more than just developing a marketable product; it also involves strict adherence to the guidelines set forth by the Food and Drug Administration (FDA) in the USA. Additionally, if your products adhere to these legal regulations, there is a high chance that educated consumers will buy them. And, of course, you won’t experience any manufacturing or marketing setbacks because of the same. This comprehensive guide will explore the FDA guidelines that must be followed when creating your supplement brand.
Understanding Dietary Supplements
Before delving into FDA guidelines, it is essential to gain an understanding of what dietary supplements actually entail. Dietary supplements encompass a diverse and vast category of products designed to improve an individual’s well-being and increase their nutritional intake. These products include vitamins, minerals, enzymes, amino acids, herbs, and more.
Unlike pharmaceutical drugs, dietary supplements are not intended to treat, prevent, or cure diseases. Instead, they are meant to complement one’s regular diet by providing essential nutrients or other compounds that may be insufficient or missing in daily nutrition. It’s important to eat a balanced diet alongside taking supplements to provide the body with a diverse array of essential nutrients and promote overall health.
Understanding this distinction is the first step in appreciating the FDA’s critical role in regulating these products to ensure their safety, efficacy, and accurate labeling.
FDA Regulations for Dietary Supplements

The U.S. Food and Drug Administration (FDA) is a federal agency in the United States responsible for regulating and overseeing the safety and efficacy of food, drugs, biologics, and other products related to public health. Its goal is to protect and promote public health by ensuring the safety and effectiveness of these products in the market.
The FDA regulates both individual dietary ingredients and finished dietary supplement products. This regulation differs from the rules governing regular food and drug products. The Dietary Supplement Health and Education Act of 1994 (DSHEA) pinpoints that:
- Manufacturers and distributors of dietary supplements and their ingredients must not promote products that are either adulterated (containing harmful substances) or misbranded (with inaccurate or misleading labels). This means that companies are responsible for assessing the safety and accuracy of their products and labels before putting them on the market, ensuring compliance with the Federal Food, Drug, and Cosmetic Act as modified by DSHEA and FDA regulations.
- The FDA holds the authority to take action against any dietary supplement product that is found to be adulterated or misbranded even after it has been introduced to the market.
FDA’s Packaging and Labeling Requirements
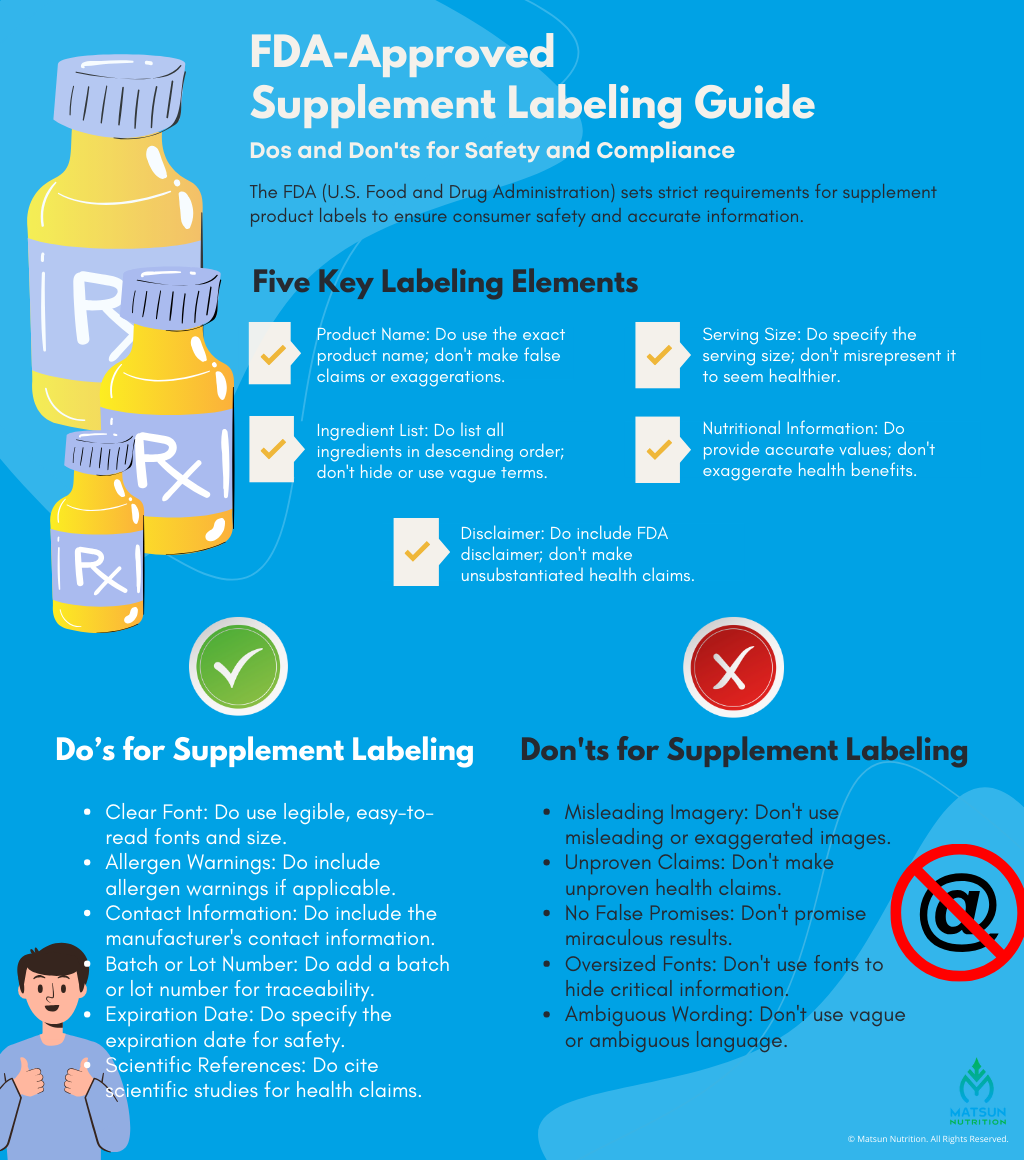
It’s crucial to ensure that your supplement’s packaging and labeling adhere to the FDA guidelines. The front label panel and the informational panel are the two places on product packaging where supplements are commonly labeled. The side that customers are most likely to view while they browse the store is the front label panel, which is usually what’s displayed on the shelf. The more technical details, such as supplement facts, ingredients, and the name and address of the producer, are all contained in the information panel, which frequently sits just to the right of the front panel. The FDA mandates that a supplement label provide five different types of information:
- The Statement of Identity – The label must clearly identify the product, including its official name. This helps consumers know what they are purchasing.
- Net Quantity of Content – The label must specify the net quantity of the product in terms of weight, volume, measure, or count.
- Nutrition Labeling – For most packaged supplements, a nutrition facts panel is required. This panel provides information about serving size, calories, nutrients, and other relevant nutrition information.
- Ingredient List – The label must include an ingredient list that lists all ingredients used to produce the supplement. This helps consumers with allergies or dietary restrictions.
- Name and Location of the Manufacturer, Distributor, or Packer – The label should provide the name and address of the manufacturer, packer, or distributor. Importers are also required to provide their information.
GMP and Its Association with Dietary Supplements
Good Manufacturing Practices (GMP) is a set of regulations and guidelines established by the FDA to ensure the quality and safety of dietary supplements. These regulations usually govern every aspect of the manufacturing process, from sourcing to testing of raw materials, record-keeping, and labeling. Compliance with GMP is mandatory for dietary supplement manufacturers, and it plays a pivotal role in safeguarding consumer health. GMP-certified manufacturers ensure that dietary supplements meet rigorous quality, safety, and consistency standards, thereby safeguarding consumer health and confidence.
New Dietary Ingredients
If a supplement brand intends to introduce a new dietary ingredient not previously marketed in the United States, it must follow the FDA’s New Dietary Ingredient (NDI) notification process. This notification aims to demonstrate the safety of the NDI for human consumption. It typically includes the scientific evidence and information about the ingredient’s history of use in dietary supplements or food. This regulatory process ensures that consumers are protected from potentially harmful or untested ingredients while allowing for innovation in the dietary supplement industry once safety can be established.
FDA Claims
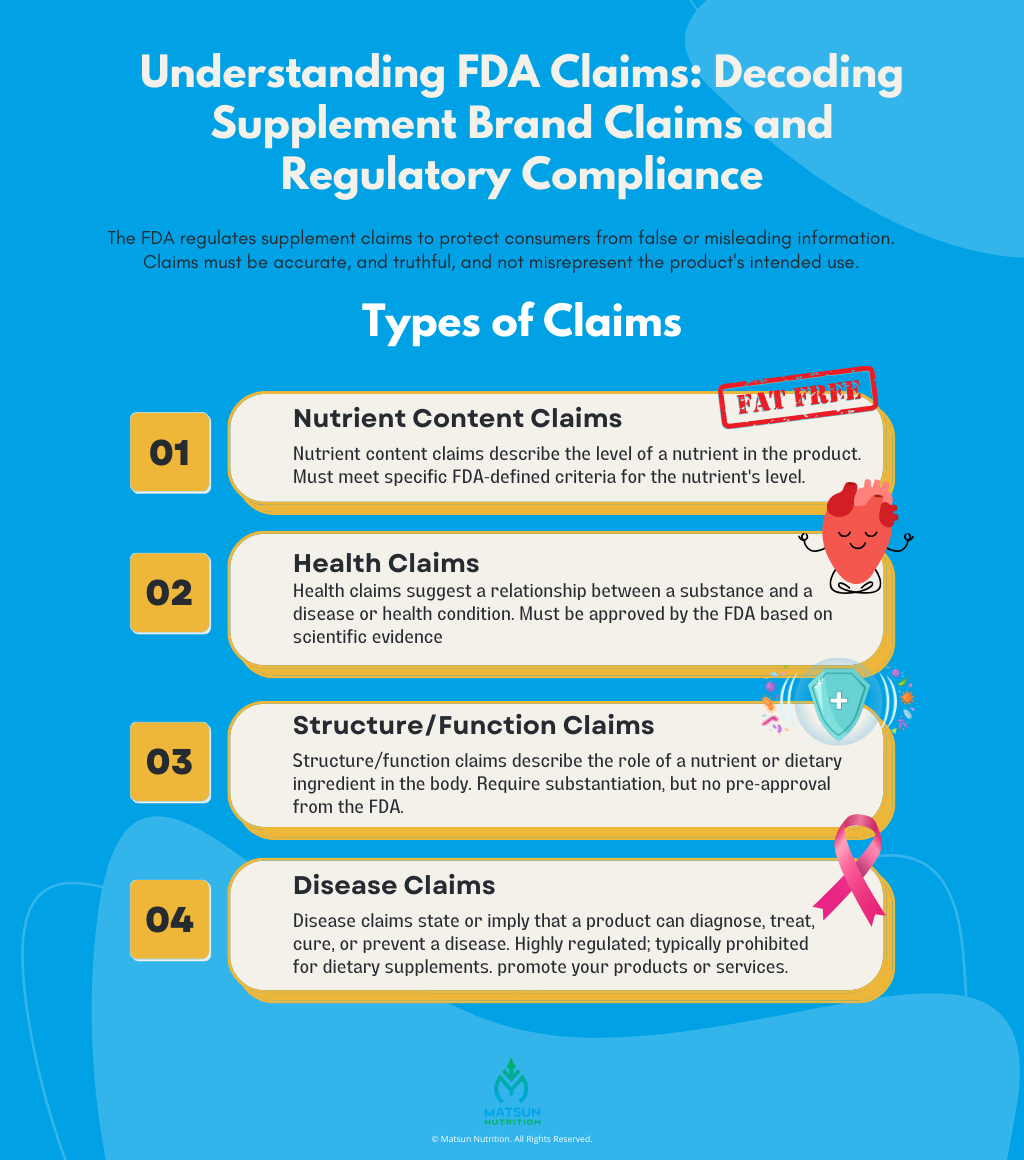
The FDA closely monitors the claims and advertising of dietary supplements. The agency categorizes claims into different types: structure/function, health claims, and nutrient content claims. Structure/function claims describe how a product may affect the body’s structure or function, but they must be truthful and not misleading. Health and nutrient content claims are subject to more stringent regulations and typically require pre-approval by the FDA. Brands must avoid making false or misleading claims about their products’ efficacy or safety. Any claims made must be supported by substantial evidence.
Adverse Events Reporting
Supplement brands are required to report Serious Adverse Events (SAEs) to the FDA. SAEs are any adverse events associated with the use of a dietary supplement that results in death, a life-threatening situation, hospitalization, disability, or other significant health problems. Compliance with adverse event reporting is crucial for consumer safety.
Warning Letters
FDA warning letters are official correspondences issued by the FDA to manufacturers, distributors, or other industry stakeholders when the agency identifies violations of regulatory standards and requirements. These letters highlight specific concerns related to product safety, labeling, manufacturing processes, or marketing claims. Warning letters serve as a means for the FDA to communicate its expectations and concerns, giving recipients an opportunity to address and rectify any issues identified.
Conclusion
Creating a successful supplement brand requires not only innovative product development and effective marketing but also strict adherence to FDA guidelines. These guidelines are in place to ensure consumer safety, product quality, and accurate labeling. By understanding and following these regulations, entrepreneurs can navigate the complex landscape of the dietary supplement industry and build a reputable and compliant brand that serves the needs of consumers while complying with FDA requirements. Suppose you’re finding it challenging to navigate these guidelines and establish your brand. In that case, it’s always a good idea to work with a reputable supplement manufacturer to help you every step of the way.
So, do you need help fulfilling those FDA requirements? Contact us today for a free consultation, and we’ll walk you through the compliance process.
Frequently Asked Questions (FAQs)
The FDA closely monitors claims made by supplement brands to ensure they are accurate and substantiated by scientific evidence. It’s essential for supplement brands to have strong support for any claims they make to avoid making unsupported or false claims.
The NDI notification process is a regulatory requirement that supplement brands must follow when introducing a new dietary ingredient into the market. This process is significant because it helps the FDA assess the safety of new ingredients, ensuring that they don’t pose health risks to consumers. By complying with the NDI notification process, supplement brands demonstrate their commitment to safety and regulatory compliance.
If you believe a dietary supplement has caused a negative effect or disease, you should first call or see your doctor immediately. After that, you or your doctor can inform the FDA about the adverse occurrence by filing a report using the Safety Reporting Portal. The identity of the reporter is kept confidential.
It is generally not legal to market a dietary supplement product with claims that it can treat, prevent, or cure a specific disease. The FDA strictly regulates the claims that can be made about dietary supplements, and a product that claims to prevent, cure, or treat a particular disease satisfies the definition of a drug and is subject to drug regulation.
Matsun Nutrition is known for its commitment to adhering to FDA guidelines and maintaining high-quality standards in supplement manufacturing. They are a GMP supplement manufacturer with a track record of full FDA compliance, meaning that their products meet the FDA’s rigorous standards for quality, safety, and consistency. The advanced quality control procedures at Matsun Nutrition make it one of the most advanced liquid supplement manufacturers in the United States.



