Choosing the right supplement manufacturer can make or break your brand. Whether you’re launching your first product line or scaling an existing business, the manufacturer you partner with will impact your product quality, compliance, timelines, and your reputation.
Not all supplement manufacturers are created equal. Some cut corners on quality testing. Others promise timelines they can’t deliver. And a few operate in regulatory gray areas that could put your entire business at risk.
Asking the right questions upfront can save you from expensive mistakes, product recalls, and damaged customer trust. In this blog, we’ll walk you through seven questions you need to ask before signing with any supplement manufacturing company, whether you’re considering a private label supplement manufacturer or exploring custom supplement manufacturing.

1. What Certifications Should a GMP Supplement Manufacturer Have?
Compliance isn’t optional in the supplement industry. The right certifications tell you whether a supplement manufacturer takes quality and safety seriously or is just checking boxes.
At minimum, your supplement manufacturer USA should be:
- FDA registered with an up-to-date facility registration
- GMP certified (Good Manufacturing Practices) through NSF, NPA, or other recognized third-party auditors
- Compliant with 21 CFR Part 111, the FDA’s dietary supplement regulations
A legitimate GMP supplement manufacturer will happily provide proof of their certifications. If they hesitate or make excuses, that’s a big red flag. These credentials represent systems, processes, and accountability that protect your brand.
Beyond the basics, ask about:
- Third-party audits and inspection schedules
- Certificate of Analysis (COA) practices
- Organic, Non-GMO, Kosher, or Halal certifications if relevant to your market
- State-specific requirements if you’re selling supplements in California, New York, or other regulated markets
Working with an FDA registered supplement manufacturer doesn’t guarantee perfection, but it does mean they’re operating within legal frameworks designed to protect consumers. Don’t compromise on compliance; it’s the foundation of everything else.
2. Understanding the Supplement Manufacturing Process: Formula to Finished Product
Understanding the supplement manufacturing process helps you gauge whether a manufacturer can truly deliver on your vision. A transparent manufacturer will walk you through each stage:
Formulation Development: Do they have in-house formulators who can help refine your concept? Can they source high-quality raw materials? How do they handle proprietary blends versus standard formulas?
Sourcing and Ingredient Verification: Where do their raw materials come from? What testing happens when ingredients arrive? Do they verify supplier credentials and request Certificates of Analysis for every batch?
Production and Quality Control: What equipment do they use? How do they prevent cross-contamination? What in-process quality checks are performed during manufacturing?
Testing and Validation: Do they conduct potency testing, microbial testing, heavy metal screening, and stability testing? Who performs these tests? In-house labs or third-party facilities?
Packaging and Labeling: Can they handle your packaging requirements? Do they review labels for regulatory compliance before printing?
A professional contract supplement manufacturer should be able to explain this entire workflow clearly. If their answers are vague or they rush through technical details, they may not have the depth of expertise your brand needs. The best supplement manufacturing services include transparency at every step.
3. Minimum Order Quantities: What to Expect From Supplement Manufacturers
MOQs (minimum order quantities) can make or break a new brand’s ability to work with certain manufacturers. Some facilities require orders of 10,000+ units, while others specialize in helping emerging brands with smaller initial runs.
When talking about MOQs with potential supplement manufacturing partners, ask:
- What’s your minimum order quantity for new clients?
- Do MOQs vary based on product format (capsules, tablets, powders, liquids)?
- Are there setup fees for custom formulations or unique packaging?
- How does pricing scale with volume?
- What payment terms do you offer?
If you’re a startup or emerging brand, finding a low MOQ supplement manufacturer can be crucial. You don’t want to invest $50,000 in inventory before you’ve validated market demand. Some private label supplement manufacturers USA offer flexible minimums specifically designed for growing brands.
Be wary of manufacturers who seem too cheap. Rock-bottom pricing often means compromised quality, inferior ingredients, or regulatory shortcuts. The goal is to find the best value for your specific needs.
4. Can Your Contract Supplement Manufacturer Produce Multiple Formats?
Your brand may start with capsules but eventually want to expand into gummies, powders, or liquid supplements. Working with a versatile custom supplement manufacturing partner saves you the headache of managing multiple vendor relationships.
Ask potential manufacturers:
- What formats can you produce? (capsules, tablets, softgels, powders, liquids, gummies, chewables)
- Do you have dedicated production lines for each format?
- Can you handle both standard and specialized formulations?
- What’s your experience with complex delivery systems like time-release or enteric coating?
For example, if you’re interested in liquid formulations, partnering with an experienced liquid vitamin manufacturer ensures they understand the unique stability challenges, flavoring requirements, and packaging considerations that liquids demand.
The ability to scale your product line without switching manufacturers is valuable. It maintains consistency, simplifies logistics, and builds on an existing relationship of trust.
5. Quality Testing Protocols Every Supplement Manufacturing Company Should Follow
Quality testing separates professional supplement manufacturers from operations that are just mixing powders. Your manufacturer should do multiple layers of testing throughout production:
Raw Material Testing: Every ingredient batch should be tested for identity, purity, potency, and contaminants before it enters production.
In-Process Testing: During manufacturing, samples should be tested to ensure consistency and proper mixing.
Finished Product Testing: Final products should undergo comprehensive testing including:
- Potency verification (do the active ingredients match the label?)
- Microbial testing (bacteria, yeast, mold, pathogens)
- Heavy metals screening (lead, arsenic, mercury, cadmium)
- Contaminant testing (pesticides, solvents, allergens)
Stability Testing: How do products perform over their shelf life? Stability studies reveal whether your supplements maintain potency and safety until expiration.
A white label supplement manufacturer worth partnering with will provide detailed testing documentation for every batch. Ask to see sample COAs (Certificates of Analysis) from previous production runs. These documents should clearly show testing methods, results, and whether the product passed specifications.

6. Evaluating a Private Label Supplement Manufacturer's Track Record
Experience matters. A manufacturer who’s produced hundreds of herbal supplements understands extraction methods, botanical ingredient challenges, and efficacy considerations that a general manufacturer might miss.
When evaluating a potential supplement manufacturing company, ask:
- Have you produced products similar to what we’re planning?
- Can you share case studies or references from current clients?
- What’s your success rate with product launches?
- Have you ever had FDA warnings, recalls, or compliance issues?
If you’re developing a specialized product, say, a mushroom extract blend or a liposomal vitamin formula, working with a manufacturer who has relevant experience dramatically reduces your risk. They’ve already solved the technical challenges you’re about to encounter.
Don’t be afraid to ask for references. Reputable manufacturers will connect you with existing clients who can speak to their experience. If a manufacturer refuses to provide any references or examples of their work, proceed with extreme caution.
7. Lead Times and Production Capabilities in Supplement Manufacturing
Even the best formulation won’t help your business if your manufacturer can’t deliver on time. Understanding production timelines prevents inventory shortages that can kill your momentum.
Key questions to ask:
- What’s your typical lead time from order to delivery?
- How far in advance do I need to place orders?
- What’s your production capacity and scheduling availability?
- Do you offer rush production for an additional fee?
- How do you handle supply chain disruptions or ingredient shortages?
Most professional supplement manufacturing services operate on 6-12 week lead times for repeat orders, with longer timelines for new product development. If a manufacturer promises unrealistically fast turnarounds, they may be overpromising.
Also talk about their capacity to grow with you. If your product takes off and you need to 10x your order volume, can they handle it? Will you get bumped down the priority list when larger clients need capacity?
A reliable nutraceutical manufacturer will be upfront about their capabilities and limitations. They’ll help you plan inventory strategically rather than making promises they can’t keep.
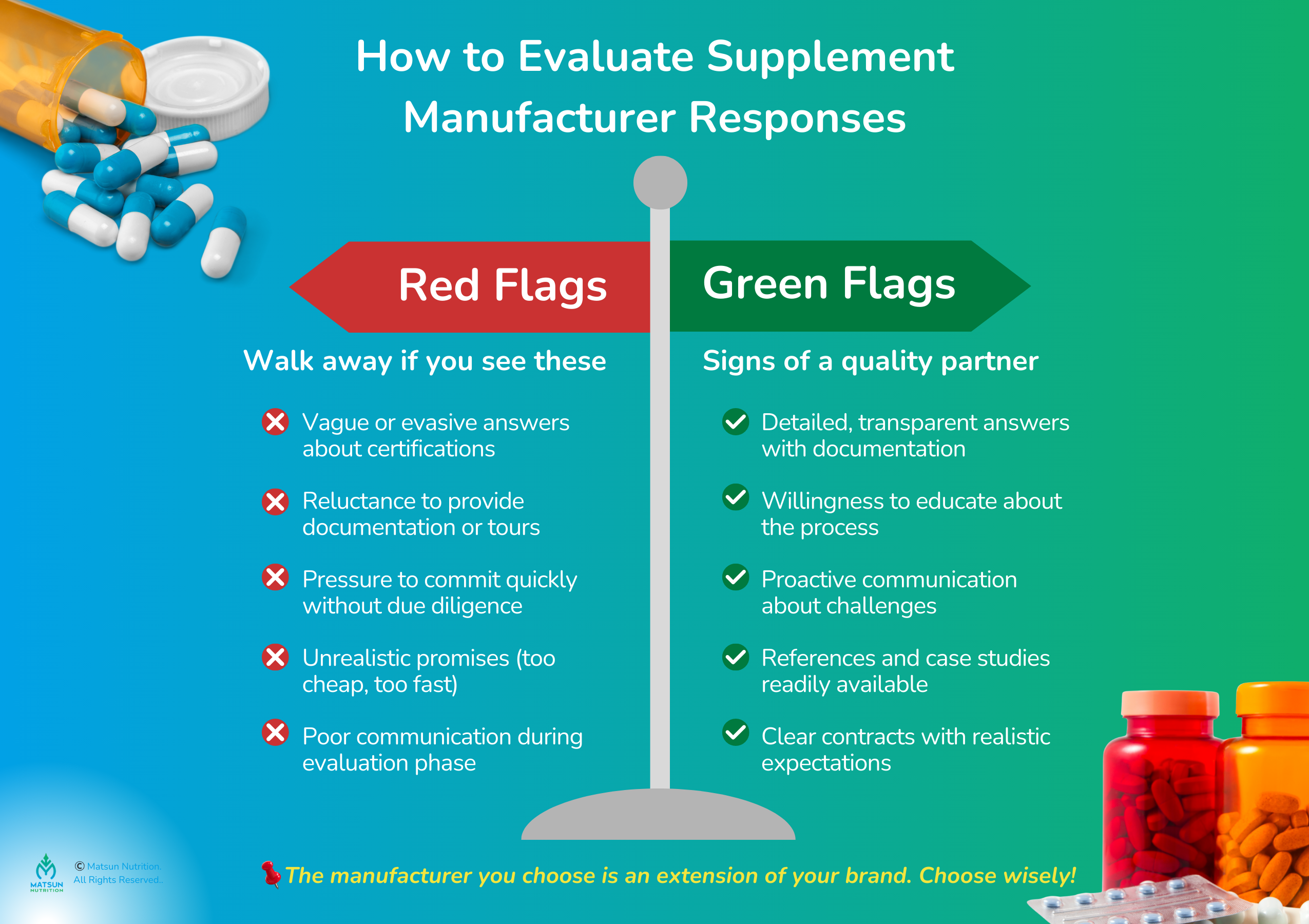
How to Evaluate Supplement Manufacturing Services Responses
Asking these questions is just the first step. The real insight comes from how manufacturers respond:
Red flags to watch for:
- Vague or evasive answers about certifications and testing
- Reluctance to provide documentation or facility tours
- Pressure to commit quickly without proper due diligence
- Promises that sound too good to be true (impossibly low prices, unrealistic timelines)
- Poor communication or slow response times during the evaluation phase
Green flags that signal a quality partner:
- Detailed, transparent answers backed by documentation
- Willingness to educate you about the supplement manufacturing process
- Proactive communication about potential challenges
- References and case studies readily available
- Clear contracts with realistic timelines and expectations
Remember, you’re choosing a partner who will represent your brand through their work. Take your time, ask follow-up questions, and trust your instincts.
Why Choosing the Right Supplement Manufacturer USA Matters
The supplement industry is growing rapidly, but it’s also highly scrutinized. One contamination issue, one mislabeled product, or one compliance violation can destroy years of brand-building work.
Your manufacturer is an extension of your brand. Their quality control becomes your quality control. Their compliance practices protect your business license. Their production capabilities determine your ability to scale.
Cutting corners during the manufacturer selection process is one of the most expensive mistakes a supplement brand can make. The few thousand dollars you might save working with a cheaper, less qualified manufacturer could cost you hundreds of thousands in recalls, legal fees, and lost customer trust.
On the other hand, partnering with the right private label supplement manufacturers creates a foundation for sustainable growth. You gain access to expertise, quality systems, and production capabilities that would cost millions to build in-house.
Making Your Final Supplement Manufacturing Company Decision
After asking these seven questions to multiple manufacturers, you’ll have the data you need to make an informed decision. Create a comparison spreadsheet that tracks:
- Certifications and compliance status
- MOQs and pricing
- Production capabilities and formats offered
- Testing protocols and documentation
- Relevant experience and references
- Lead times and capacity
- Overall communication and professionalism
The “best” manufacturer isn’t always the biggest or most expensive. It’s the one whose capabilities, values, and communication style align with your brand’s needs and growth trajectory.
For emerging brands, finding a partner who offers reasonable MOQs, strong quality systems, and responsive communication often matters more than working with an industry giant who views you as a small account.
For scaling brands, production capacity, format versatility, and proven experience with similar products might be the deciding factors.
Trust the process. Do your due diligence. And remember that switching manufacturers later is expensive and disruptive, it’s worth investing the time to choose right the first time.
Ready to work with a supplement manufacturer who answers these questions with confidence and transparency? At Matsun Nutrition, we’re committed to quality, compliance, and partnership. Explore our supplement manufacturer services, get a custom quote for low MOQ options, or browse our liquid vitamin manufacturing and nutraceutical manufacturing capabilities to see the quality we deliver. Let’s build something great together.
Frequently Asked Questions
What questions should I ask a supplement manufacturer before hiring?
Ask about their certifications (GMP, FDA registration), testing protocols, MOQs, production capabilities, lead times, experience with similar products, and their complete manufacturing process. Request documentation for everything and speak with current client references before making a decision.
How do I verify a supplement manufacturer’s certifications?
Ask for copies of their GMP certificate, FDA registration, and any third-party audit reports. You can verify FDA registration by searching the FDA’s database online. For GMP certification, confirm with the certifying body (NSF, NPA, etc.) that the certificate is current and valid.
What is private label supplement manufacturing?
Private label manufacturing is when a manufacturer produces supplements using their existing formulations, which you then sell under your own brand name and label. It’s faster and more cost-effective than custom formulation, making it ideal for new brands testing the market.
Are USA-based supplement manufacturers better for compliance?
USA-based manufacturers must comply with FDA regulations and are subject to domestic inspections, which generally provides stronger oversight. Domestic manufacturers also offer easier communication, faster shipping, and reduced import/export complications, though qualified international manufacturers can also meet high standards.
What is a reasonable MOQ for supplement manufacturing?
MOQs vary widely by manufacturer and product type. New brands should look for manufacturers offering MOQs between 1,000-5,000 units. Some specialized low-MOQ manufacturer starts as low as 500-1,000 units or less, while larger operations may require 10,000+ units per order. Matsun Nutrition offers Low MOQs supplement manufacturing services with 2500 bottles.
How long does supplement manufacturing take?
For repeat orders, expect 6-12 weeks from order to delivery. New product development takes longer; typically 12-20 weeks including formulation, testing, packaging design, and production. Rush orders may be available for additional fees, but plan inventory carefully to avoid shortages.
Can one manufacturer produce multiple supplement formats?
Many full-service manufacturers can produce capsules, tablets, powders, and liquids, though not all offer every format. If you plan to expand your product line into different formats, confirm your manufacturer has the equipment, expertise, and dedicated production lines for each format you’ll need.
What testing should a supplement manufacturer provide?
At minimum, manufacturers should provide raw material testing (identity, purity, potency), in-process testing during production, and finished product testing (potency verification, microbial testing, heavy metals screening, and contaminant testing). All results should be documented in Certificates of Analysis (COAs).
How do I avoid common supplement manufacturing mistakes?
Do thorough due diligence before selecting a manufacturer. Verify certifications, review testing protocols, check references, and tour facilities when possible. Start with smaller orders to test quality before scaling. Build strong communication channels and have clear contracts that outline expectations, timelines, and quality standards.
What’s the difference between private label and custom supplements?
Private label uses the manufacturer’s existing formulations with your branding, offering faster time-to-market and lower development costs. Custom manufacturing creates unique formulations specific to your brand, providing differentiation and proprietary products but requiring more time, higher MOQs, and greater investment in development.




