The dietary supplements industry is a goldmine, but it’s also a regulatory minefield. For brands and manufacturers, mastering dietary supplement regulations is critical to avoid costly missteps and build a reputation that customers trust.
At Matsun Nutrition, we’ve guided countless brands through the maze of supplement compliance guidelines, and we’re here to share practical, human-sounding advice. From FDA supplement regulations to supplement import/export rules, this guide breaks down the essentials to keep your business compliant and thriving. Let’s dive into the world of dietary supplement manufacturing rules and set your brand up for success.
Understanding Dietary Supplement Regulations
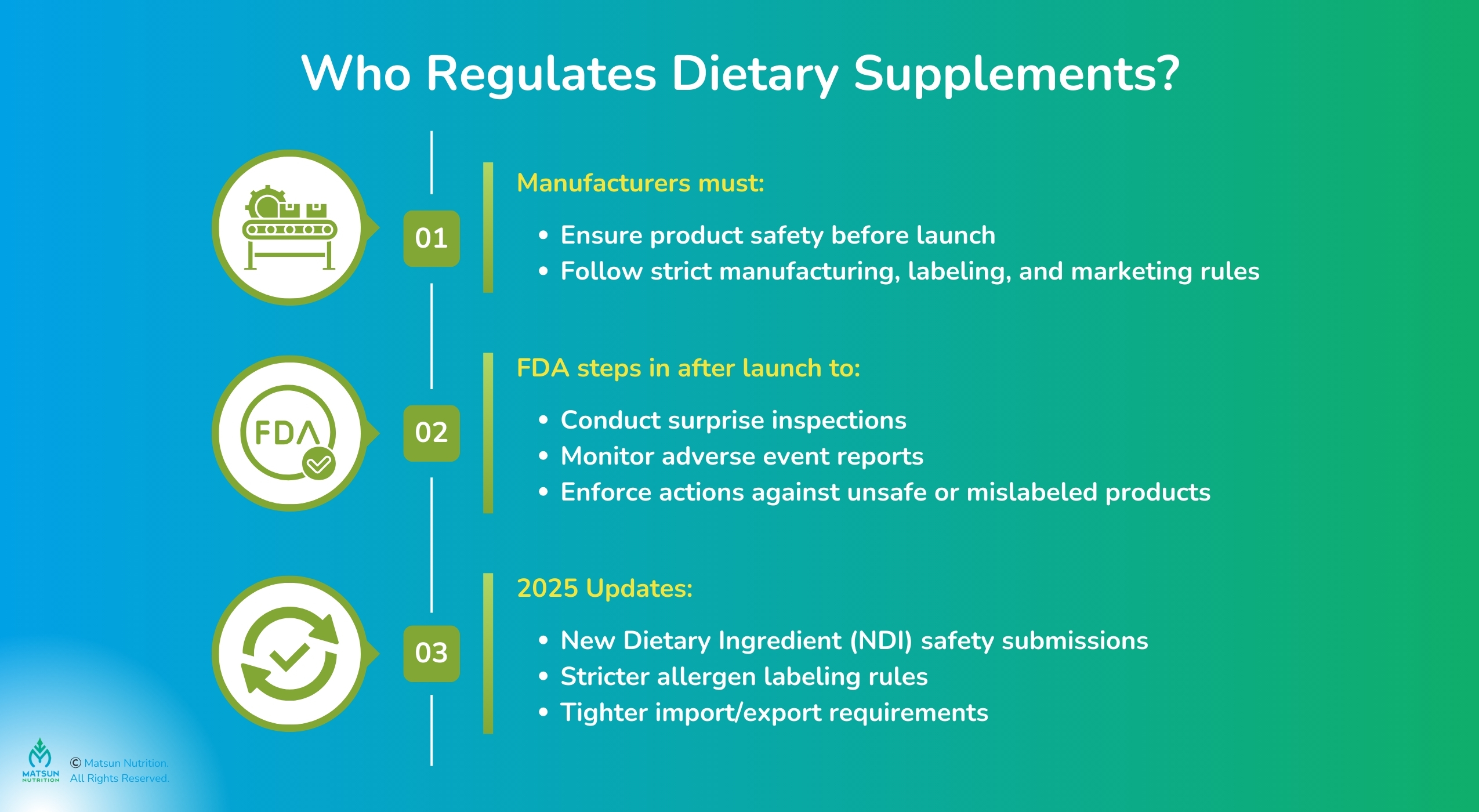
Dietary supplement regulations govern products like vitamins, herbs, and protein powders, which primarily fall under the Dietary Supplement Health and Education Act (DSHEA) of 1994. Under DSHEA, supplements are regulated as food, not drugs, meaning the manufacturer is responsible for ensuring safety before launch, while the FDA handles post-market monitoring.
In 2025 and 2026, this responsibility is sharper than ever. The FDA has finalized key guidance on the New Dietary Ingredient (NDI) Notification process. This clarifies the required market-ready documentation you need to submit at least 75 days before launch, including evidence of safety and specific product manufacturing details. Supplement production regulations also cover cGMP manufacturing, accurate labeling, and marketing. Staying ahead means having a partner who ensures your NDI submissions and compliance documentation are flawless. This guide will show you how.
Why Compliance Matters for Dietary Supplement Brands
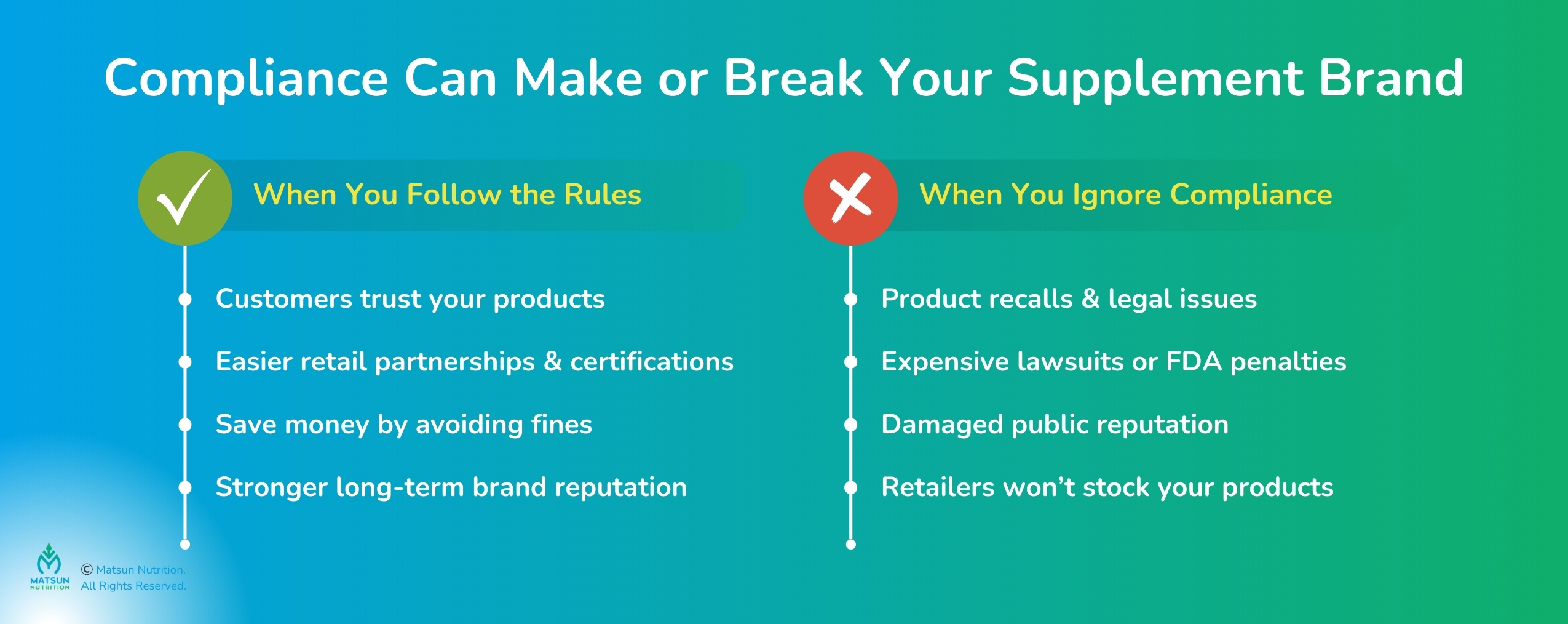
Supplement industry compliance isn’t just a legal hoop to jump through; it’s the backbone of a trustworthy brand. Customers in 2025 are savvy. They demand transparency and proof of supplement safety standards. Ignoring supplement compliance guidelines can lead to recalls, lawsuits, or a PR disaster that damages your reputation. I’ve seen brands crumble over a single mislabeled product.
On the flip side, nailing vitamin manufacturing compliance sets you apart. Retailers often require proof of compliance before stocking your products. Certifications from the supplement certification process (like NSF or USP) boost credibility. Compliance also saves cash. Fines and legal battles are brutal. At Matsun Nutrition, we’ve watched compliant brands ace audits and win loyal customers. As one industry expert said, “Compliance is your brand’s foundation for trust and growth.” It’s not just about following supplement business regulations, it’s about building a legacy.
FDA Oversight in Supplement Manufacturing
FDA supplement regulations put the responsibility on manufacturers to ensure safety before products hit the market, with the FDA stepping in for post-market oversight. This includes:
- Inspections: The FDA can conduct surprise facility inspections to check for supplement manufacturing standards.
- Adverse Event Reports: The FDA collects and monitors reports of negative health outcomes linked to supplements.
- Enforcement: Issues like poor records or contamination risks can trigger warning letters or seizures.
In 2025, the FDA’s Human Foods Program is tightening supplement distribution rules, with stricter import requirements and allergen labeling standards. If you’re using a new ingredient, the supplement product approval process requires an NDI notification with safety data. Partnering with an FDA-registered manufacturer like Matsun Nutrition takes the heat off, as we are already compliant with the latest requirements.
GMP Standards Every Brand Should Follow
Good Manufacturing Practices (GMP) are the heart of GMP for supplements, outlined in the FDA’s 21 CFR Part 111. These supplement manufacturing rules ensure your facility, staff, and equipment meet supplement safety standards to prevent contamination or mix-ups.
To be GMP-compliant, you’ll need to:
- Test raw materials for identity and purity.
- Vet all suppliers and keep detailed batch records for traceability.
- Maintain a clean production environment with calibrated machines.
The supplement certification process with groups like NSF or USP adds credibility and market appeal. At Matsun Nutrition, GMP is our core. See how we do it at our low MOQ supplement manufacturer. Following GMP for supplements isn’t just about compliance; it’s about making products you’d trust yourself.

Labeling Rules for Dietary Supplements
Supplement labeling requirements are strict to keep customers informed and prevent deception. Your “Supplement Facts” panel must list serving size, ingredients, and amounts in clear units (like milligrams), plus allergen declarations for things like soy or wheat. Supplement packaging rules also require your contact info for adverse event reports.
Every label needs the FDA disclaimer: “This statement has not been evaluated by the FDA. This product is not intended to diagnose, treat, cure, or prevent any disease.” Mess this up, and you’re risking a misbranding charge.
For example, a vitamin C label might say “Vitamin C (as ascorbic acid) 90 mg” with the disclaimer. At Matsun Nutrition, we help brands perfect their labels because compliant supplement packaging rules build trust and keep regulators happy.
Why Matsun Nutrition Holds These Certifications (and What It Means for You)
At Matsun Nutrition, we don’t just talk about compliance — we live it. Over more than 25 years in supplement manufacturing, we’ve built our processes to meet and exceed key certifications and industry standards, ensuring your products are built on a foundation of trust, safety, and quality.
Here’s how our certifications back up our promise to you:
| Certification / Standard | What It Means | Benefit to Your Brand |
|---|
| GMP (Good Manufacturing Practices, e.g. 21 CFR Part 111) | We follow rigorous internal controls, testing, documentation, and facility standards. | Reduces risk of contamination, mix-ups, or recalls — helps you approach compliance confidently. |
| FDA-Registered Facility / Compliance with DSHEA | We are up to date with the latest FDA rules for dietary supplements and ingredient approvals. | Saves you time and worry — you don’t have to reinvent compliance from scratch. |
| Third-Party Testing / Quality Controls | We routinely test raw materials and finished products (identity, potency, contaminants). | Provides credible documentation you can share with retailers or regulators. |
| Labeling & Claims Oversight | Our regulatory team reviews your label copy, claims, and marketing to ensure compliance. | Helps protect your brand from misbranding or misleading claims concerns. |
| Export / Global Regulatory Readiness | We monitor international supplement regulations to support global expansion. | When you scale internationally, your formulations and packaging are already positioned to adapt. |
Because we maintain these standards on your behalf, partnering with us means your brand inherits that compliance stability from day one. Instead of scrambling to secure certifications and worry about audit gaps, you can focus on formulation, branding, and market strategy.
If you’re ready to bring your supplement vision to life — with full confidence in compliance — we’d love to help. Get a quote from Matsun Nutrition today to see how we can support your next product launch with certified, high-quality manufacturing service.
Approved and Prohibited Supplement Claims
Supplement claims regulations let you make structure/function claims like “supports immune health,” but you can’t claim your product fights specific diseases. That’s a hard pass. Every claim needs solid evidence and the FDA disclaimer. The FTC also watches your ads for supplement marketing compliance, ensuring they’re not misleading.
Avoid These Prohibited Terms:
- “Cures”
- “Prevents”
- “Treats”
Stick to safe, science-backed wellness claims. Getting supplement claims regulations right keeps your brand safe and your customers confident.
Quality Control in Supplement Production
Supplement quality control is your safety net, ensuring every step, from raw materials to finished products, meets supplement safety standards. Test raw ingredients for purity, monitor production for consistency, and check final products for potency and contaminants. A single bad batch can lead to recalls or customer harm, so don’t cut corners.
Batch testing and stability studies are musts, and third-party testing adds extra trust. At Matsun Nutrition, we’re obsessive about supplement quality control. Strong quality control isn’t just about meeting supplement production regulations; it’s about delivering products that live up to your brand’s promise.
Global Regulations for Exporting Supplements
Taking your dietary supplements global? Supplement import/export rules vary widely.
What You Need to Know:
- The EU requires novel food approvals.
- Canada demands Natural Health Product licenses.
- U.S. brands need FDA export certificates and must adapt labels for each market.
In 2025, trade agreements are pushing for stricter supplement distribution rules, like proving GMP equivalence or detailed safety data. One mistake can halt your shipment. Work with regulatory experts early to navigate supplement import/export rules.
Common Compliance Mistakes to Avoid
Brands often trip over simple stuff: mislabeling, skipping GMP for supplements, or sloppy record-keeping. Other pitfalls include using unverified suppliers, ignoring adverse event reports, or making risky claims. These can lead to FDA crackdowns or customer backlash.
Fix it with regular audits, staff training, and a solid supplement compliance guidelines checklist. Learn from others’ mistakes; it’s cheaper than fixing your own. Stay proactive to keep regulators and customers happy.
Preparing for a Supplement Facility Audit
An FDA audit can feel like a high-stakes exam, but prep makes it manageable. Update your Standard Operating Procedures (SOPs), run internal audits, and train your team on supplement manufacturing standards. Keep records organized, because inspectors love clarity. Fix past issues, like equipment gaps, before they arrive.
Mock audits are your best friend; they catch weak spots early. At Matsun Nutrition, we’ve helped brands turn audits into a chance to shine. A prepared facility doesn’t just pass, it impresses.
Ready to build a compliant, high-quality dietary supplements brand? Partner with Matsun Nutrition for top-tier manufacturing. Let’s talk about your vision and make it happen.
RELATED READ:
How To Find Low MOQ Supplement Manufacturer for SMEs – Comprehensive Guide
FAQs
What are dietary supplement regulations?
Dietary supplement regulations under DSHEA and the FDA ensure dietary supplements are safe, properly labeled, and marketed truthfully. They also establish Good Manufacturing Practices and outline enforcement actions for noncompliance.
Who regulates dietary supplements in the U.S.?
The FDA oversees dietary supplements in the U.S., handling post-market checks, inspections, and adverse event reports. The FTC also enforces truth-in-advertising standards for marketing claims.
How do GMP rules affect supplement brands?
GMP for supplements ensures safe, consistent production, helping brands avoid recalls and build trust with retailers and customers. They require documented procedures, validated equipment, and routine quality control testing.
What labeling rules must supplements follow?
Supplement labeling requirements mandate a Supplement Facts panel, allergen info, and an FDA disclaimer for clear, honest labels. Labels cannot claim to diagnose, treat, cure, or prevent any disease.
Can supplements make health benefit claims?
Yes, structure/function claims like “supports immunity” are allowed under supplement claims regulations if backed by evidence and disclaimed. Disease claims are prohibited, and any health claims must meet specific FDA criteria.
How do I prepare for a compliance audit?
Update SOPs, train staff, organize records, and run mock audits to meet supplement manufacturing standards confidently. Assign an audit lead and verify supplier certifications and COAs are current.
What happens if a brand violates regulations?
Violating supplement business regulations can lead to FDA warnings, seizures, fines, or recalls, plus reputational damage. Severe or repeated violations can result in consent decrees or criminal liability.
Are global supplement regulations different?
Yes, supplement import/export rules vary, with regions like the EU and Canada requiring unique approvals and labeling. Research destination-country requirements early to prevent delays or relabeling.





